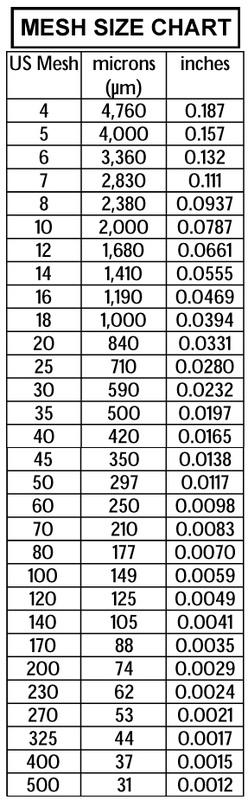
Resource: Cameron Carbon Incorporated
Activated carbon can be considered as a material of phenomenal surface area made up of millions of pores rather like a “molecular sponge”.
The process by which such a surface concentrates fluid molecules by chemical and/or physical forces is known as ADSORPTION (whereas, ABSORPTION is a process whereby fluid molecules are taken up by a liquid or solid and distributed throughout that liquid or solid).
The process by which such a surface concentrates fluid molecules by chemical and/or physical forces is known as ADSORPTION (whereas, ABSORPTION is a process whereby fluid molecules are taken up by a liquid or solid and distributed throughout that liquid or solid).
In the physical adsorption process, molecules are held by the carbon’s surface by weak forces known as Van Der Waals Forces resulting from intermolecular attraction. The carbon and the adsorbate are thus unchanged chemically. However, in the process known as CHEMISORPTION molecules chemically react with the carbon’s surface (or an impregnate on the carbon’s surface) and are held by much stronger forces chemical bond.
In general terms, to affect adsorption it is necessary to present the molecule to be adsorbed to a pore of comparable size. In this way the attractive forces coupled with opposite wall effect will be at a maximum and should be greater than the energy of the molecule.
For example, a fine pored coconut shell carbon has poor decolorizing properties because color molecules tend to be larger molecular species and are thus denied access to a fine pore structure. In contrast, coconut shell carbons are particularly efficient in adsorbing small molecular species. Krypton and Xenon, for instance, are readily adsorbed by coconut shell carbon but readily desorbs from large pored carbons such as wood.
Maximum adsorption capacity is determined by the degree of liquid packing that can occur in the pores. In very high vapor pressures, multilayer adsorption can lead to capillary condensation even in macrospores (25A).
If adsorption capacity is plotted against pressure (for gases) or concentration (for liquids) at constant temperature, the curve so produced is known as an ISOTHERM.
Adsorption increases with increased pressure and also with increasing molecular weight, within a series of a chemical family. Thus, methane (CH4) is less easily adsorbed than propane (C3H8).
This is a useful fact to remember when a particular system has a number of components.
After equilibrium, it is generally found that, all else being equal; the higher molecular weight species of a multi-component system are preferentially adsorbed. Such a phenomenon is known as competitive or preferential adsorption - the initially adsorbed low molecular weight species desorbing from the surface and being replaced by higher molecular weight species.
Physical adsorption in the vapor phase is affected by certain external parameters such as temperature and pressure. The adsorption process is more efficient at lower temperatures and higher pressures since molecular species are less mobile under such conditions. Such an effect is also noticed in a system where moisture and an organic species are present. The moisture is readily accepted by the carbon surface but in time desorbs as the preferred organic molecules are selected by the surface. This usually occurs due to differences in molecular size but can be also attributable to the difference in molecular charge.
Generally speaking, carbon surfaces dislike any form of charge - since water is highly charged (ionic) relative to the majority of organic molecules the carbon would prefer the organic to be adsorbed. Primary amines possess less charge on the nitrogen atom than secondary amines that in turn have less than tertiary amines. Thus, it is found that primary amines are more readily adsorbed than tertiary amines.
High levels of adsorption can be expected if the adsorbate is a reasonably large bulky molecule with no charge, whereas a small molecule with high charge would not be expected to be easily adsorbed.
Molecular shape also influences adsorption but this is usually of minor consideration.
In certain situations, regardless of how the operating conditions can be varied, some species will only be physically adsorbed to a low level. (Examples are ammonia, sulfur dioxide, hydrogen sulfide, and mercury vapor and methyl iodide). In such instances, the method frequently employed to enhance a carbon’s capability is to impregnate it with a particular compound that is chemically reactive towards the species required to be adsorbed.
Since carbon possesses such a large surface (a carbon granule the size of a “quarter” has a surface area in the order of ½ square mile!) coating of this essentially spreads out the impregnate over a vast area. This therefore, greatly increases the chance of reaction since the adsorbate has a tremendous choice of reaction sites. When the adsorbate is removed in this way the effect is known as CHEMISORPTION.
Unlike physical adsorption the components of the system are changed chemically and the changed adsorbate chemically held by the carbon’s surface and adsorption in the original form is nonexistent. This principle is applied in many industries, particularly in the catalysis field, where the ability of a catalyst can be greatly increased by spreading it over a carbon surface.
In general terms, to affect adsorption it is necessary to present the molecule to be adsorbed to a pore of comparable size. In this way the attractive forces coupled with opposite wall effect will be at a maximum and should be greater than the energy of the molecule.
For example, a fine pored coconut shell carbon has poor decolorizing properties because color molecules tend to be larger molecular species and are thus denied access to a fine pore structure. In contrast, coconut shell carbons are particularly efficient in adsorbing small molecular species. Krypton and Xenon, for instance, are readily adsorbed by coconut shell carbon but readily desorbs from large pored carbons such as wood.
Maximum adsorption capacity is determined by the degree of liquid packing that can occur in the pores. In very high vapor pressures, multilayer adsorption can lead to capillary condensation even in macrospores (25A).
If adsorption capacity is plotted against pressure (for gases) or concentration (for liquids) at constant temperature, the curve so produced is known as an ISOTHERM.
Adsorption increases with increased pressure and also with increasing molecular weight, within a series of a chemical family. Thus, methane (CH4) is less easily adsorbed than propane (C3H8).
This is a useful fact to remember when a particular system has a number of components.
After equilibrium, it is generally found that, all else being equal; the higher molecular weight species of a multi-component system are preferentially adsorbed. Such a phenomenon is known as competitive or preferential adsorption - the initially adsorbed low molecular weight species desorbing from the surface and being replaced by higher molecular weight species.
Physical adsorption in the vapor phase is affected by certain external parameters such as temperature and pressure. The adsorption process is more efficient at lower temperatures and higher pressures since molecular species are less mobile under such conditions. Such an effect is also noticed in a system where moisture and an organic species are present. The moisture is readily accepted by the carbon surface but in time desorbs as the preferred organic molecules are selected by the surface. This usually occurs due to differences in molecular size but can be also attributable to the difference in molecular charge.
Generally speaking, carbon surfaces dislike any form of charge - since water is highly charged (ionic) relative to the majority of organic molecules the carbon would prefer the organic to be adsorbed. Primary amines possess less charge on the nitrogen atom than secondary amines that in turn have less than tertiary amines. Thus, it is found that primary amines are more readily adsorbed than tertiary amines.
High levels of adsorption can be expected if the adsorbate is a reasonably large bulky molecule with no charge, whereas a small molecule with high charge would not be expected to be easily adsorbed.
Molecular shape also influences adsorption but this is usually of minor consideration.
In certain situations, regardless of how the operating conditions can be varied, some species will only be physically adsorbed to a low level. (Examples are ammonia, sulfur dioxide, hydrogen sulfide, and mercury vapor and methyl iodide). In such instances, the method frequently employed to enhance a carbon’s capability is to impregnate it with a particular compound that is chemically reactive towards the species required to be adsorbed.
Since carbon possesses such a large surface (a carbon granule the size of a “quarter” has a surface area in the order of ½ square mile!) coating of this essentially spreads out the impregnate over a vast area. This therefore, greatly increases the chance of reaction since the adsorbate has a tremendous choice of reaction sites. When the adsorbate is removed in this way the effect is known as CHEMISORPTION.
Unlike physical adsorption the components of the system are changed chemically and the changed adsorbate chemically held by the carbon’s surface and adsorption in the original form is nonexistent. This principle is applied in many industries, particularly in the catalysis field, where the ability of a catalyst can be greatly increased by spreading it over a carbon surface.