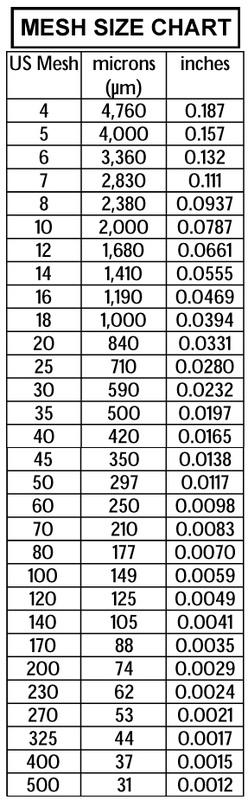
Resource: Cameron Carbon Incorporated
The first known use of activated carbon dates back to the Ancient Egyptians who utilized its adsorbent properties for purifying oils and medicinal purposes. Centuries later, the early ocean-going vessels stored drinking water in wooden barrels, the inside of which had been charred. (However, by modern definition the carbon used in these applications could not truly be described as “activated”). By the early 19th century both wood and bone charcoal was in large-scale use for the decolorization and purification of cane sugar.