Resource: Cameron Carbon Incorporated
Because of the diverse end uses to which a carbon may be applied, it is difficult for manufacturers to conduct specific tests related to any one application. A manufacturer can undertake some specialty tests after agreement with the user but this is the exception rather than the rule. The size and number of pores essentially determine a carbon’s capacity in adsorbing a specific compound. Since pore size and total pore volume determinations are quite lengthy, they are impractical as a means of quality control during manufacture. It is, therefore, necessary to relate the carbon’s surface capabilities to a standard reference molecule.
Carbon Tetrachloride Activity
The most widely used method is to measure the carbon’s capacity to adsorb carbon tetrachloride (referred to as CTC) and express this as a w/w %. This is determined by flowing CTC laden air through a sample of carbon of known weight, under standard conditions, until constant weight is achieved. The apparatus essentially consists of a means to control the supply of air pressure, produce a specified concentration of CTC and control the flow rate of the air/CTC mixture through the sample. The weight of CTC adsorbed is referred to as the carbon’s % CTC activity. However, this test does not necessarily provide an absolute or relative measure of the effectiveness of the carbon for other adsorbents or under different conditions. CTC activity is now universally accepted as a means of specifying the degree of activation or quality of activated carbon. Commercially available carbons range from 20% to 90% CTC activity.
Surface Area
The internal surface area of a carbon is usually determined by the BET method (Brunauer, Emmett and Teller). This method utilizes the low-pressure range of the adsorption isotherm of a molecule of known dimensions (usually nitrogen). This region of the isotherm is generally attributed to monolayer adsorption. Thus, by assuming the species is adsorbed only one molecule deep on the carbon’s surface, the surface area may be calculated using the equation:
S =XmNA / M
S = specific surface in m2/g
Xm = sorption value (weight of adsorbed N2 divided by weight of carbon sample)
N = Avogadro’s Number, 6.025 E+23
A = cross-sectional area of nitrogen molecule in angstroms
M = molecular weight of nitrogen
Most manufacturers will specify the surface area of their products but as with CTC activity, it does not necessarily provide a measure of their effectiveness, merely demonstrating their degree of activation. It is also impractical to utilize surface area measurement as a means of quality control since this is a very lengthy procedure.
S =XmNA / M
S = specific surface in m2/g
Xm = sorption value (weight of adsorbed N2 divided by weight of carbon sample)
N = Avogadro’s Number, 6.025 E+23
A = cross-sectional area of nitrogen molecule in angstroms
M = molecular weight of nitrogen
Most manufacturers will specify the surface area of their products but as with CTC activity, it does not necessarily provide a measure of their effectiveness, merely demonstrating their degree of activation. It is also impractical to utilize surface area measurement as a means of quality control since this is a very lengthy procedure.
Hardness
The hardness and resistance to attrition of activated carbons is becoming more and more important.
The loosely applied term of “hardness” is somewhat difficult to measure on activated carbon.
Three forces can mechanically degrade an activated carbon - impact, crushing and attrition.
Of these three, the force of attrition, or abrasion, is the most common cause of degradation in actual end use.
At the present time, there are two commonly used methods available to evaluate a carbon’s hardness.
The first of these is the Ball-pan Hardness Test.
A screened, weighed sample of carbon is placed in a special hardness pan with a number of stainless steel balls and subjected to combined rotating and tapping action for ½ hour. The particle size degradation is measured by determining the weight of carbon retained on a sieve (with an opening closest to one half the opening of the sieve defining the minimum nominal particle size of the original sample). The ball-pan hardness method has been used widely in the past and has a broad history in the activated carbon industry for measuring the property loosely described as “hardness”. In this context, the test is useful in establishing a measurable characteristic, conceding that it does not actually measure in-service resistance to degradation, it can be used to establish comparability of differing batches of the same material. This test actually applies all of the three forces mentioned earlier, in a variable manner determined by the size, shape and density of the particles.
The second method used is the Stirring Bar Abrasion Test.
In this procedure, a sample of carbon is placed in a cylindrical vessel where an inverted T-shaped stirrer is turning rapidly at a controlled rate. The percentage reduction in average particle size, resulting from the T-bar action, is recorded after 1 hour. This method measures attrition of the carbon, as long as the particle size is smaller than a 12 mesh. There is evidence showing that the results of this method are influenced by particle geometry.
Whichever of these tests is performed on carbon it is generally accepted that granular coconut based carbons show the least rate of physical degradation.
This is possible due to two factors. First, granular coconut carbon is produced from pieces of raw coconut shell whereas; most other carbons are produced from reconstituted powders. In consequence, carbons other than coconut based types can only breakdown to a powder or dust.
Coconut carbon essentially chips and breaks into smaller pieces and thus degradation to powder, is a relatively lengthy process. Second, as outlined earlier, the coconut carbon structure is different to other types, producing a material of relatively high density and physical strength.
The loosely applied term of “hardness” is somewhat difficult to measure on activated carbon.
Three forces can mechanically degrade an activated carbon - impact, crushing and attrition.
Of these three, the force of attrition, or abrasion, is the most common cause of degradation in actual end use.
At the present time, there are two commonly used methods available to evaluate a carbon’s hardness.
The first of these is the Ball-pan Hardness Test.
A screened, weighed sample of carbon is placed in a special hardness pan with a number of stainless steel balls and subjected to combined rotating and tapping action for ½ hour. The particle size degradation is measured by determining the weight of carbon retained on a sieve (with an opening closest to one half the opening of the sieve defining the minimum nominal particle size of the original sample). The ball-pan hardness method has been used widely in the past and has a broad history in the activated carbon industry for measuring the property loosely described as “hardness”. In this context, the test is useful in establishing a measurable characteristic, conceding that it does not actually measure in-service resistance to degradation, it can be used to establish comparability of differing batches of the same material. This test actually applies all of the three forces mentioned earlier, in a variable manner determined by the size, shape and density of the particles.
The second method used is the Stirring Bar Abrasion Test.
In this procedure, a sample of carbon is placed in a cylindrical vessel where an inverted T-shaped stirrer is turning rapidly at a controlled rate. The percentage reduction in average particle size, resulting from the T-bar action, is recorded after 1 hour. This method measures attrition of the carbon, as long as the particle size is smaller than a 12 mesh. There is evidence showing that the results of this method are influenced by particle geometry.
Whichever of these tests is performed on carbon it is generally accepted that granular coconut based carbons show the least rate of physical degradation.
This is possible due to two factors. First, granular coconut carbon is produced from pieces of raw coconut shell whereas; most other carbons are produced from reconstituted powders. In consequence, carbons other than coconut based types can only breakdown to a powder or dust.
Coconut carbon essentially chips and breaks into smaller pieces and thus degradation to powder, is a relatively lengthy process. Second, as outlined earlier, the coconut carbon structure is different to other types, producing a material of relatively high density and physical strength.
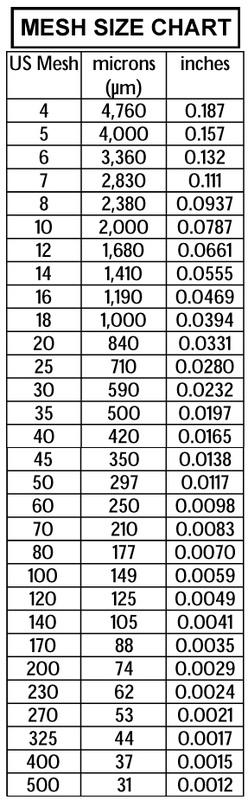
Mesh Size
The physical size, or mesh size, of a carbon must be considered in relation to the flow rate in the system it is to be used.
Naturally, the smaller the carbon’s mesh size, the greater its resistance to flow. Thus, it is usual to select the smallest mesh size carbon that will satisfy the pressure drop limitations of the system.
Naturally, the smaller the carbon’s mesh size, the greater its resistance to flow. Thus, it is usual to select the smallest mesh size carbon that will satisfy the pressure drop limitations of the system.
Ash Content
Ash content is less important except where the carbon is used as a catalyst support since certain constituents of the ash may interfere or destroy the action of precious metal catalysts. Ash content also influences the ignition point of the carbon—this may be a major consideration where adsorption of certain solvents is concerned.
Density
The density of carbon is, of course, of great importance to many users in estimating the weight required to fill a vessel.
Interrelation of Properties
There is a relationship between BET surface area and CTC adsorption and this is taken into account when specifications are formulated. CTC activity, density and ash content are interrelated and provide a simple means of manufacturing control.
As quality, or degree of activation increases, CTC activity and ash content increase and density decreases.
Furthermore, CTC activity being equal, coconut carbons show higher density and lower ash content than coal based carbons. Wood based carbons show much lower density than either coal or coconut carbons but ash contents midway between coal and coconut carbons.
Thus, these properties are not only a means of controlling quality during manufacture but may also assist in determining the raw material and quality of an unknown carbon.
CTC activity, density, hardness, mesh size and raw material information will enable selection of a suitable carbon for most common applications (excepting those utilizing chemisorptions as the prime mechanism).
As quality, or degree of activation increases, CTC activity and ash content increase and density decreases.
Furthermore, CTC activity being equal, coconut carbons show higher density and lower ash content than coal based carbons. Wood based carbons show much lower density than either coal or coconut carbons but ash contents midway between coal and coconut carbons.
Thus, these properties are not only a means of controlling quality during manufacture but may also assist in determining the raw material and quality of an unknown carbon.
CTC activity, density, hardness, mesh size and raw material information will enable selection of a suitable carbon for most common applications (excepting those utilizing chemisorptions as the prime mechanism).
Other Tests
Many other tests such as methylene blue, iodine number, molasses number, phenol value, ABS (alkyl benzene sulfonate) value etc., can be carried out but these are usually only relevant to specific end-uses.