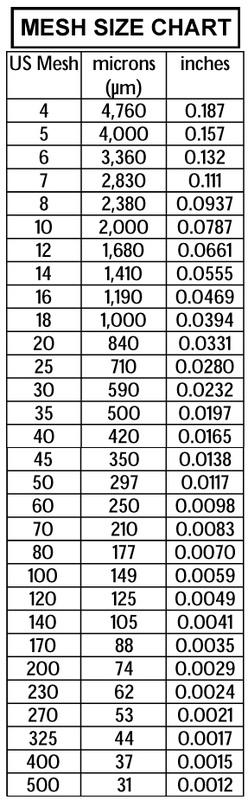
Resource: Cameron Carbon Incorporated
Raw Materials
It has already been stated that essentially any carbonaceous material can potentially be activated. In addition to the more common raw materials discussed earlier, others can include waste tires, phenol formaldehyde resin, rice husks, pulp mill residues, corn cobs, coffee beans and bones.
Present total annual world production capacity is estimated at 300,000 tons: available as granular, extruded or powdered product.
Most of the developed nations have facilities to activate coconut shell, wood and coal. Third world countries have recently entered the industry and concentrate on readily available local raw materials such as wood and coconut shell.
Coconut shell contains about 75% volatile matter that is removed, largely at source by partial carbonization, to minimize shipping costs. The cellulosic structure of the shell determines the end product characteristics, which (at 30-40% yield on the carbonized basis) is a material of very high internal surface area consisting of pores and capillaries of fine molecular dimensions. The ash content is normally low and composed mainly of alkalis and silica.
Coal is also a readily available and reasonably cheap raw material. The activate obtained depends on the type of coal used and its initial processing prior to carbonization and activation.
It is normal procedure to grind the coal and reconstitute it into a form suitable for processing, by use of a binder such as pitch, before activation. (This is typical for extruded or pelletized carbon). An alternative method is to grind the coal and utilize its volatile content to fuse the powder together in the form of a briquette. This method allows for blending of selected materials to control the swelling power of the coals and prevents coking. If the coal is allowed to “coke” it leads to the production of an activate with an unacceptably high proportion of large pores. Blending of coals also allows a greater degree of control over the structure and properties of the final product.
Wood may be activated by one of two methods, i.e. steam or chemical activation, depending on the desired product. A common chemical activator is phosphoric acid, which produces a char with a large surface area suitable for decolorization applications. The carbon is usually supplied as a finely divided powder which since produced from waste materials such as sawdust, is relatively cheap and can be used on a “throw-away” basis.
Since activated carbon is manufactured from naturally occurring raw materials, its properties will obviously be variable. In order to minimize variability it is necessary to be very selective in raw material source and quality and practice a high level of manufacturing quality control.
Most of the developed nations have facilities to activate coconut shell, wood and coal. Third world countries have recently entered the industry and concentrate on readily available local raw materials such as wood and coconut shell.
Coconut shell contains about 75% volatile matter that is removed, largely at source by partial carbonization, to minimize shipping costs. The cellulosic structure of the shell determines the end product characteristics, which (at 30-40% yield on the carbonized basis) is a material of very high internal surface area consisting of pores and capillaries of fine molecular dimensions. The ash content is normally low and composed mainly of alkalis and silica.
Coal is also a readily available and reasonably cheap raw material. The activate obtained depends on the type of coal used and its initial processing prior to carbonization and activation.
It is normal procedure to grind the coal and reconstitute it into a form suitable for processing, by use of a binder such as pitch, before activation. (This is typical for extruded or pelletized carbon). An alternative method is to grind the coal and utilize its volatile content to fuse the powder together in the form of a briquette. This method allows for blending of selected materials to control the swelling power of the coals and prevents coking. If the coal is allowed to “coke” it leads to the production of an activate with an unacceptably high proportion of large pores. Blending of coals also allows a greater degree of control over the structure and properties of the final product.
Wood may be activated by one of two methods, i.e. steam or chemical activation, depending on the desired product. A common chemical activator is phosphoric acid, which produces a char with a large surface area suitable for decolorization applications. The carbon is usually supplied as a finely divided powder which since produced from waste materials such as sawdust, is relatively cheap and can be used on a “throw-away” basis.
Since activated carbon is manufactured from naturally occurring raw materials, its properties will obviously be variable. In order to minimize variability it is necessary to be very selective in raw material source and quality and practice a high level of manufacturing quality control.
Methods of Manufacture
Activated carbon can be produced by either steam or chemical activation, both of which require the use of elevated temperature.
Chemical activation is achieved by degradation or dehydration of the, usually cellulosic, raw material structure. Steam activation, however, initially involves the removal of volatiles, followed by oxidation of the structure’s carbon atoms.
Chemical activation is achieved by degradation or dehydration of the, usually cellulosic, raw material structure. Steam activation, however, initially involves the removal of volatiles, followed by oxidation of the structure’s carbon atoms.
Chemical Activation
The raw material used in chemical activation is usually sawdust and the most popular activating agent is phosphoric acid, although zinc chloride and sulfuric acid are well documented. Others used in the past include calcium hydroxide, calcium chloride, manganese chloride and sodium hydroxide, all of which are dehydrating agents.
The raw material and reagent are mixed into a paste, dried and carbonized in a rotary furnace at 600 degrees C. When phosphoric acid is the activating agent the carbonized product is further heated at 8001000 degrees C during which stage the carbon is oxidized by the acid. The acid is largely recovered after the activation stage and converted back to the correct strength for reuse.
The activated product is washed with water and dried.
Activity can be controlled by altering the proportion of raw material to activating agent, between the limits of 1:05 to 1:4. By increasing the concentration of the activating agent, the activity increases although control of furnace temperature and residence time can achieve the same objective.
The raw material and reagent are mixed into a paste, dried and carbonized in a rotary furnace at 600 degrees C. When phosphoric acid is the activating agent the carbonized product is further heated at 8001000 degrees C during which stage the carbon is oxidized by the acid. The acid is largely recovered after the activation stage and converted back to the correct strength for reuse.
The activated product is washed with water and dried.
Activity can be controlled by altering the proportion of raw material to activating agent, between the limits of 1:05 to 1:4. By increasing the concentration of the activating agent, the activity increases although control of furnace temperature and residence time can achieve the same objective.
Steam Activation
The use of steam for activation can be applied to virtually all raw materials.
A variety of methods have been developed but all of these share the same basic principle of initial carbonization at 500-600 degrees C followed by activation with steam at 800-1,100 degrees C.
Since the overall reaction (converting carbon to carbon dioxide) is exothermic it is possible to utilize this energy and have a self-sustaining process.
C + H2O (steam) ---> CO + H2 (-31 Kcal)
CO + ½ O2 ---> CO2 (+67 Kcal)
H2 + ½ O2 ---> H2O (steam) (+58 Kcal)
C + O2 ---> CO2 (+94 Kcal)
A number of different types of kilns and furnaces can be used for carbonization/activation and include rotary (fired directly or indirectly), vertical multi-hearth furnaces, fluidized bed reactors and vertical single throat retorts. Each manufacturer has their own preference.
As an example, production of activated carbon using a vertical retort is described below.
Raw material is introduced through a hopper on top of the retort and falls under gravity through a central duct towards the activation zone. As the raw material moves slowly down the retort the temperature increases to 800-1000 degrees C and full carbonization takes place.
The activation zone, at the bottom of the retort, covers only a small part of the total area available and it is here that steam activation takes place. Air is bled into the furnace to convert the product gases, CO and H2 into CO2 and steam which, because of the exothermic nature of this reaction, reheats the firebricks on the downside of the retort, enabling the process to be self-supporting.
Every 15 minutes or so, the steam injection point is alternated to utilize the “in situ” heating provided by the product gas combustion. The degree of activation (or quality) of the product is determined by the residence time in the activation zone.
The resulting product is in the form of 1” to 3” pieces and requires further processing before being suitable for its various end uses. This entails a series of crushing and screening operations to produce specific mesh ranges.
Certain products may undergo further processing such as drying, acid washing or chemical impregnation to satisfy particular requirements.
A variety of methods have been developed but all of these share the same basic principle of initial carbonization at 500-600 degrees C followed by activation with steam at 800-1,100 degrees C.
Since the overall reaction (converting carbon to carbon dioxide) is exothermic it is possible to utilize this energy and have a self-sustaining process.
C + H2O (steam) ---> CO + H2 (-31 Kcal)
CO + ½ O2 ---> CO2 (+67 Kcal)
H2 + ½ O2 ---> H2O (steam) (+58 Kcal)
C + O2 ---> CO2 (+94 Kcal)
A number of different types of kilns and furnaces can be used for carbonization/activation and include rotary (fired directly or indirectly), vertical multi-hearth furnaces, fluidized bed reactors and vertical single throat retorts. Each manufacturer has their own preference.
As an example, production of activated carbon using a vertical retort is described below.
Raw material is introduced through a hopper on top of the retort and falls under gravity through a central duct towards the activation zone. As the raw material moves slowly down the retort the temperature increases to 800-1000 degrees C and full carbonization takes place.
The activation zone, at the bottom of the retort, covers only a small part of the total area available and it is here that steam activation takes place. Air is bled into the furnace to convert the product gases, CO and H2 into CO2 and steam which, because of the exothermic nature of this reaction, reheats the firebricks on the downside of the retort, enabling the process to be self-supporting.
Every 15 minutes or so, the steam injection point is alternated to utilize the “in situ” heating provided by the product gas combustion. The degree of activation (or quality) of the product is determined by the residence time in the activation zone.
The resulting product is in the form of 1” to 3” pieces and requires further processing before being suitable for its various end uses. This entails a series of crushing and screening operations to produce specific mesh ranges.
Certain products may undergo further processing such as drying, acid washing or chemical impregnation to satisfy particular requirements.