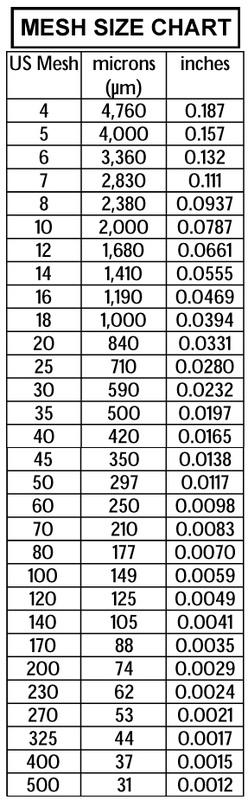
Resource: Cameron Carbon Incorporated
Almost all materials containing a high fixed carbon content can potentially be activated. The most commonly used raw materials are coal (anthracite, bituminous and lignite), coconut shells, wood (both soft and hard), peat and petroleum based residues.
Many other raw materials have been evaluated such as walnut shells, peach pits, babassu nutshell and palm kernels but invariably their commercial limitation lies in raw material supply. This is illustrated by considering that 1,000 tons of untreated shell type raw material will only yield about 100 tons of good quality activated carbon.
Most carbonaceous materials do have a certain degree of porosity and an internal surface area in the range of 10-15 m2/g. During activation, the internal surface becomes more highly developed and extended by controlled oxidation of carbon atoms - usually achieved by the use of steam at high temperature.
After activation, the carbon will have acquired an internal surface area between 700 and 1,200 m2/g, depending on the plant operating conditions.
The internal surface area must be accessible to the passage of a fluid or vapor if a potential for adsorption is to exist. Thus, it is necessary that an activated carbon has not only a highly developed internal surface but accessibility to that surface via a network of pores of differing diameters.
As a generalization, pore diameters are usually categorized as follows:
micropores <40 Angstroms
mesopores 40 - 5,000 Angstroms
macropores >5,000 Angstroms (typically 5000-20000 A)
During the manufacturing process, macropores are first formed by the oxidation of weak points (edge groups) on the external surface area of the raw material. Mesopores are then formed and are, essentially, secondary channels formed in the walls of the macropore structure. Finally, the micropores are formed by attack of the planes within the structure of the raw material.
All activated carbons contain micropores, mesopores, and macropores within their structures but the relative proportions vary considerably according to the raw material.
A coconut shell based carbon will have a predominance of pores in the micropore range and these account for 95% of the available internal surface area. Such a structure has been found ideal for the adsorption of small molecular weight species and applications involving low contaminant concentrations.
In contrast wood and peat based carbons are predominantly meso/ macrospore structures and are, therefore, usually suitable for the adsorption of large molecular species. Such properties are used to advantage in decolonization processes.
Coal based carbons, depending on the type of coal used, contain pore structures somewhere between coconut shell and wood.
In general, it can be said that macrospores are of little value in their surface area, except for the adsorption of unusually large molecules and are, therefore, usually considered as an access point to microspores.
Macrospores do not generally play a large role in adsorption, except in particular carbons where the surface area attributable to such pores is appreciable (usually 400 m2/g or more).
Thus, it is the microspore structure of an activated carbon that is the effective means of adsorption.
It is, therefore, important that activated carbon not be classified as a single product but rather a range of products suitable for a variety of specific applications.
Most carbonaceous materials do have a certain degree of porosity and an internal surface area in the range of 10-15 m2/g. During activation, the internal surface becomes more highly developed and extended by controlled oxidation of carbon atoms - usually achieved by the use of steam at high temperature.
After activation, the carbon will have acquired an internal surface area between 700 and 1,200 m2/g, depending on the plant operating conditions.
The internal surface area must be accessible to the passage of a fluid or vapor if a potential for adsorption is to exist. Thus, it is necessary that an activated carbon has not only a highly developed internal surface but accessibility to that surface via a network of pores of differing diameters.
As a generalization, pore diameters are usually categorized as follows:
micropores <40 Angstroms
mesopores 40 - 5,000 Angstroms
macropores >5,000 Angstroms (typically 5000-20000 A)
During the manufacturing process, macropores are first formed by the oxidation of weak points (edge groups) on the external surface area of the raw material. Mesopores are then formed and are, essentially, secondary channels formed in the walls of the macropore structure. Finally, the micropores are formed by attack of the planes within the structure of the raw material.
All activated carbons contain micropores, mesopores, and macropores within their structures but the relative proportions vary considerably according to the raw material.
A coconut shell based carbon will have a predominance of pores in the micropore range and these account for 95% of the available internal surface area. Such a structure has been found ideal for the adsorption of small molecular weight species and applications involving low contaminant concentrations.
In contrast wood and peat based carbons are predominantly meso/ macrospore structures and are, therefore, usually suitable for the adsorption of large molecular species. Such properties are used to advantage in decolonization processes.
Coal based carbons, depending on the type of coal used, contain pore structures somewhere between coconut shell and wood.
In general, it can be said that macrospores are of little value in their surface area, except for the adsorption of unusually large molecules and are, therefore, usually considered as an access point to microspores.
Macrospores do not generally play a large role in adsorption, except in particular carbons where the surface area attributable to such pores is appreciable (usually 400 m2/g or more).
Thus, it is the microspore structure of an activated carbon that is the effective means of adsorption.
It is, therefore, important that activated carbon not be classified as a single product but rather a range of products suitable for a variety of specific applications.