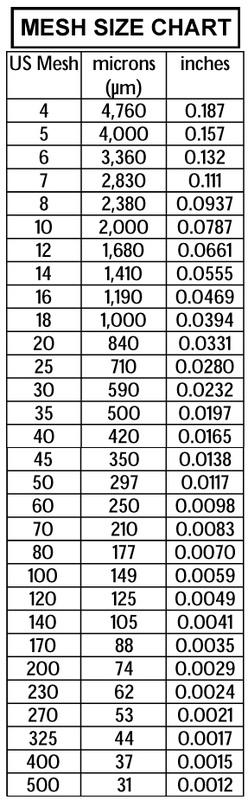
Resource: Cameron Carbon Incorporated
Surface Exhaustion
In almost every application of activated carbon the surface will eventually become saturated (or exhausted). This may occur within a few weeks, several months or many years depending on the conditions of service.
Saturation is inevitable since the carbon surface has a finite number of reaction sites and when these are all occupied an adsorption potential no longer exists.
Carbon is a very non-selective sorbet and has a great affinity for a wide spectrum of organic compounds. Although, a carbon may be designed and employed to remove a very specific compound from a process stream in doing so it will undoubtedly adsorb most other components in the stream thus creating a cumulative affect on the rate and degree of saturation. In fact if so called "clean air" were to be passed through a carbon filter it would eventually become saturated, even though the concentration of contaminants may be in the ppb range.
Carbon is a very non-selective sorbet and has a great affinity for a wide spectrum of organic compounds. Although, a carbon may be designed and employed to remove a very specific compound from a process stream in doing so it will undoubtedly adsorb most other components in the stream thus creating a cumulative affect on the rate and degree of saturation. In fact if so called "clean air" were to be passed through a carbon filter it would eventually become saturated, even though the concentration of contaminants may be in the ppb range.
Surface Regeneration
In many applications (utilizing base, non-impregnated carbon) the surface can be regenerated or reactivated in-situ, using steam or other heat treatment processes, thus allowing reuse of the carbon many times over. This principle is used to great advantage in the recovery of volatile organic solvents ..... the desorbed contaminants (i.e. solvents) being recovered from the steam used to strip the carbon surface.
In situations where the adsorbed contaminants are not readily desorbed by steam, thermal reactivation in a kiln (similar to that used in the activation process) is necessary.
In the case of chemically impregnated carbons, reactivation is seldom possible since the chemisorbed contaminants are chemically fixed on the carbon’s surface and the impregnate cannot be returned to its original state.
Although the predominant mechanism of an impregnated carbon is one of chemisorptions, some physical adsorption, to varying degrees, will also take place. In theory the physically adsorbed species could be removed from a saturated impregnated carbon by reactivation. However, the reactivated carbon would remain inactive towards those species requiring a chemisorptions removal mechanism since the impregnate would either still be exhausted or be chemically changed by the reactivation conditions.
In situations where the adsorbed contaminants are not readily desorbed by steam, thermal reactivation in a kiln (similar to that used in the activation process) is necessary.
In the case of chemically impregnated carbons, reactivation is seldom possible since the chemisorbed contaminants are chemically fixed on the carbon’s surface and the impregnate cannot be returned to its original state.
Although the predominant mechanism of an impregnated carbon is one of chemisorptions, some physical adsorption, to varying degrees, will also take place. In theory the physically adsorbed species could be removed from a saturated impregnated carbon by reactivation. However, the reactivated carbon would remain inactive towards those species requiring a chemisorptions removal mechanism since the impregnate would either still be exhausted or be chemically changed by the reactivation conditions.